Whilst looking for what the second generation Lemtrada, which I think may be abit of media spin, I had a quick look at trials and the patents Genzyme and Sanofi have filed.
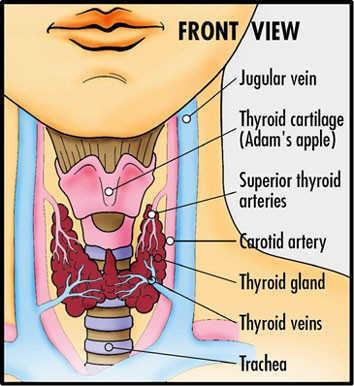
One of the major side-effects of Alemtuzumab treatment is the development of thyroid disease, where they developed antibodies against the thyrotropin receptor and this cause carbimazole-responsive autoimmune hyperthyroidism. (An overactive thyroid) This occurred in about a third of MSers (Coles AJ et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354:1691.
One of the major side-effects of Alemtuzumab treatment is the development of thyroid disease, where they developed antibodies against the thyrotropin receptor and this cause carbimazole-responsive autoimmune hyperthyroidism. (An overactive thyroid) This occurred in about a third of MSers (Coles AJ et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354:1691.
In addition there were other secondary autoimmune conditions in smaller numbers. This autoimmunity typically arises during reconstitution of the lymphocyte repertoire.
The A's from Cambridge did a study and suggested that individuals with high baseline circulating levels of interleukin 21 (IL-21) were at a higher risk of developing autoimmunity following treatment with alemtuzumab. Jones JL et al. Interleukin-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest. 2009119:2052-61.
The Guys from Genzyme think there is something else that increases risk of developing thyroid autoimmunity and this is pre-existing antibodies to thyroid peroxidase.
Margolin D METHOD OF IDENTIFYING RISK FOR THYROID DISORDER. W02008103292.
A method for identifying a patient that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes lymphocytes, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at increased risk for developing a thyroid disorder. A particular embodiment is a method for identifying a patient with multiple sclerosis that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes CD52-positive cells, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at risk for developing the thyroid disorder.
Of the MSers that did not have baseline thyroid peroxidase antibodies 19% (n=35/182) went on to develop thyroid disease and 25% had thyroid stimulating hormone receptor antibodies, in contrast of those positive at baseline, 50% developed thyroid disease (n=8/16) and 65% developed thyroid stimulating hormone receptor antibodies. These could result in thyroid disease.
The A's from Cambridge did a study and suggested that individuals with high baseline circulating levels of interleukin 21 (IL-21) were at a higher risk of developing autoimmunity following treatment with alemtuzumab. Jones JL et al. Interleukin-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest. 2009119:2052-61.
The Guys from Genzyme think there is something else that increases risk of developing thyroid autoimmunity and this is pre-existing antibodies to thyroid peroxidase.
Margolin D METHOD OF IDENTIFYING RISK FOR THYROID DISORDER. W02008103292.
A method for identifying a patient that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes lymphocytes, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at increased risk for developing a thyroid disorder. A particular embodiment is a method for identifying a patient with multiple sclerosis that is at risk for developing a thyroid disorder that occurs subsequent to treatment with a regimen that depletes CD52-positive cells, comprising determining whether antibodies directed against thyroid peroxidase or thyroid microsomes are present in the patient, wherein if the antibodies are present in the patient then the patient is at risk for developing the thyroid disorder.
Of the MSers that did not have baseline thyroid peroxidase antibodies 19% (n=35/182) went on to develop thyroid disease and 25% had thyroid stimulating hormone receptor antibodies, in contrast of those positive at baseline, 50% developed thyroid disease (n=8/16) and 65% developed thyroid stimulating hormone receptor antibodies. These could result in thyroid disease.
As is always the case you have to treat patents with a dose of skepticism because there is no peer-review of the information, so you can claim loads of things. It is really only lawyers that assess the merits and patents and is on legal aspects like novelty and invention and not whether the data is good quality. However the presence of thyroid peroxidase antibody is known to be a risk factor for the development of thyroid disease, and MSers are no different. Food for thought if you ever get the option to try it.
Pharma do loads of work that never sees the light of day in the academic literature, but this surfaces in the patent database